38 label these groups of the periodic table.
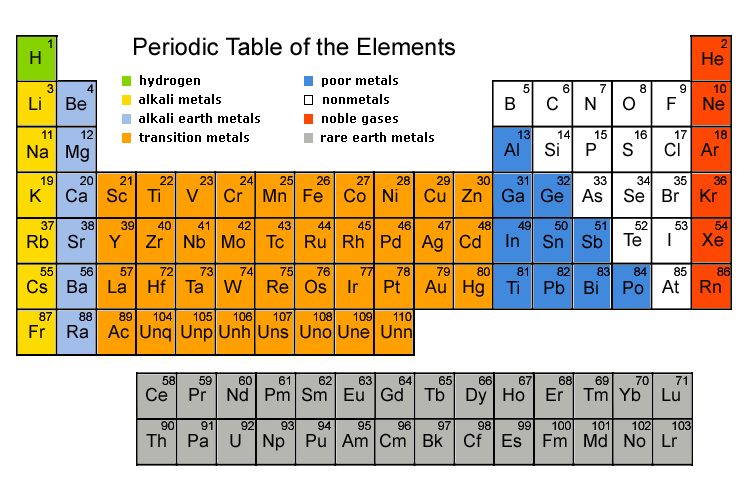
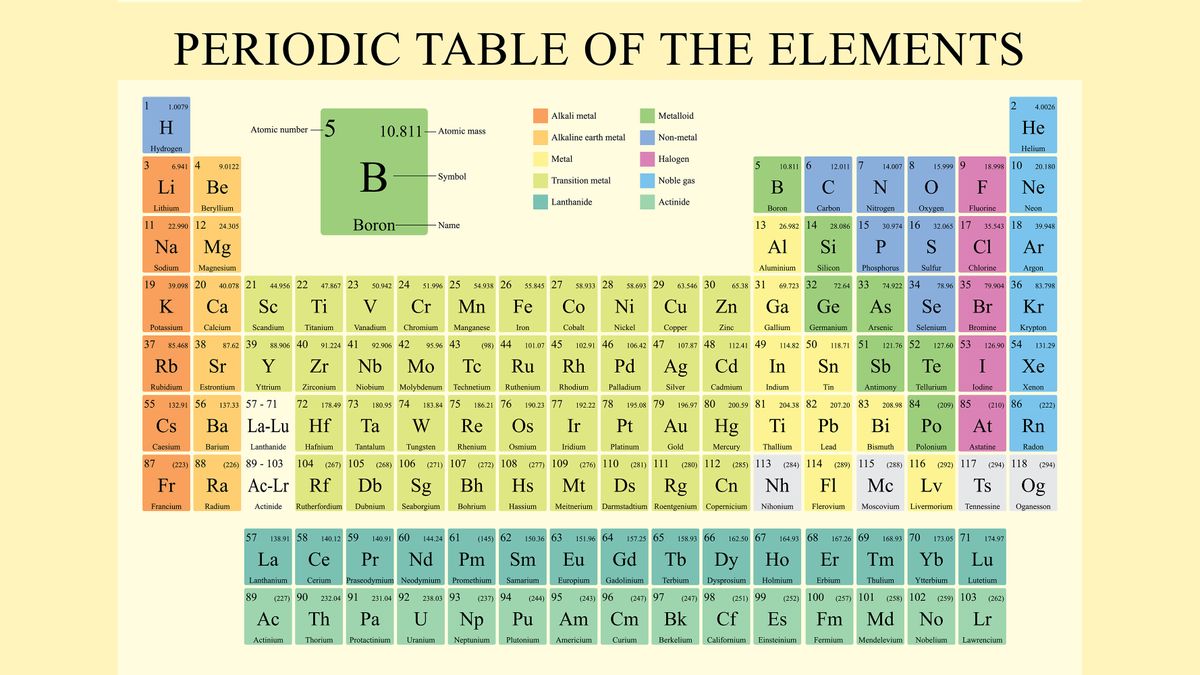
Labeling_the_Periodic_Table (1).pdf - Labeling the Periodic... Metalloids are found in groups3A-7A. They are Boron, Silicon, Germanium, Arsenic, Antimony, Tellurium and Astatine. They are also called semi-metals. Color these all the same color. 5. Nonmetals a. Noble Gases (also called Inert Gases)b. Halogens (the halogens are the elements in group 7A)c. Nonmetals in groups 4A-6A d. Hydrogen 6. OneClass: Label these groups of the periodic table. Rank from largest to smallest atomic radius. To rank items as equivalent, overlap them. The periodic table lists all known elements by their masses and groups elements by their properties. Within the periodic table, there are many properties of the elements that have periodic trends as we move down the groups in the table or across the periods.
periodic table | Definition, Elements, Groups, Charges, Trends, & Facts ... periodic table, in full periodic table of the elements, in chemistry, the organized array of all the chemical elements in order of increasing atomic number—i.e., the total number of protons in the atomic nucleus. When the chemical elements are thus arranged, there is a recurring pattern called the "periodic law" in their properties, in which elements in the same column (group) have ...
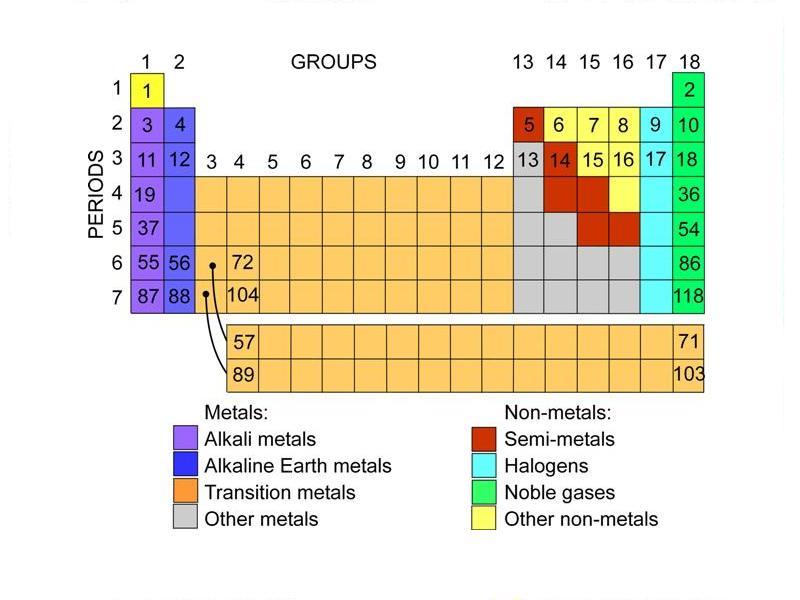
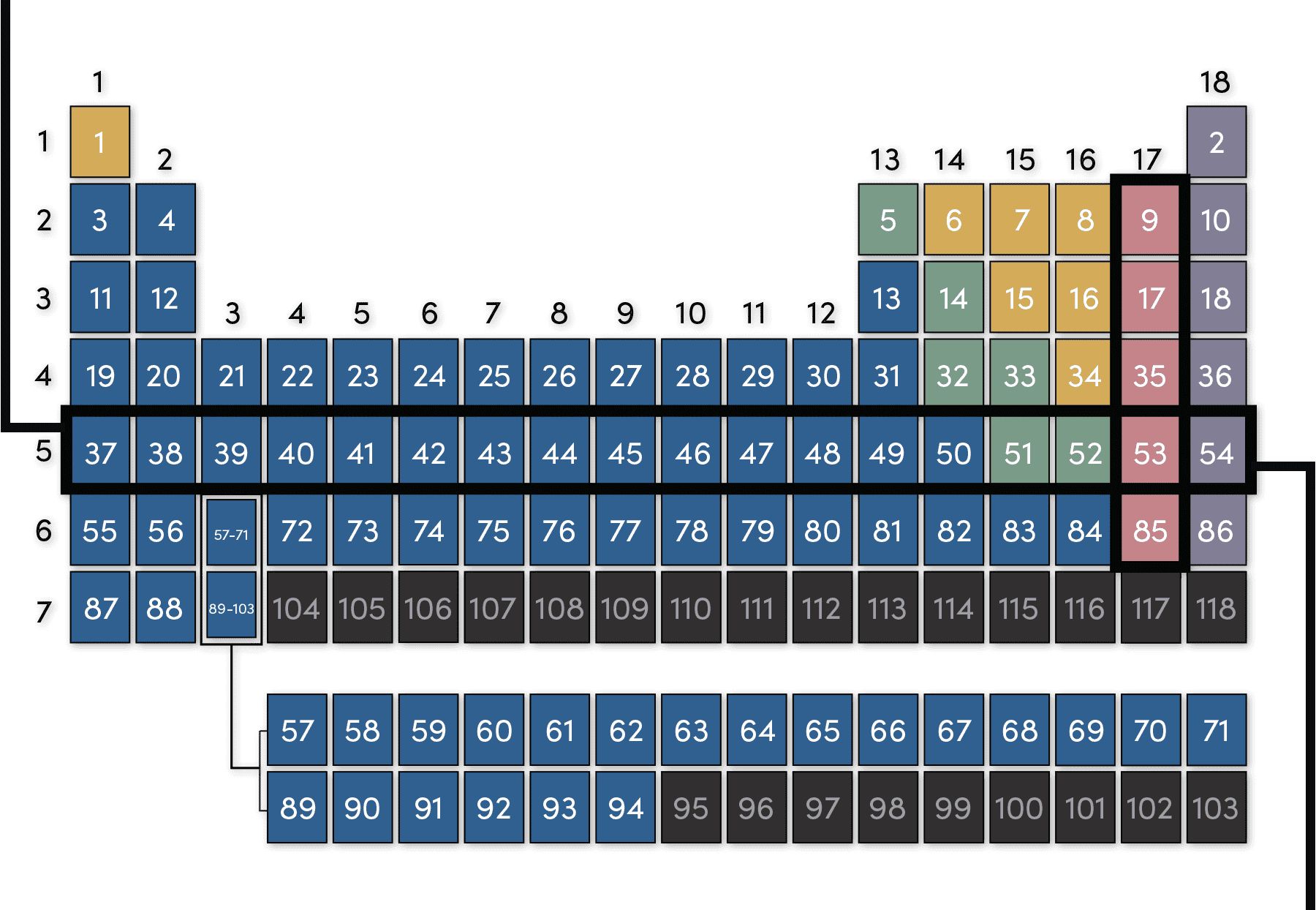
Label these groups of the periodic table.
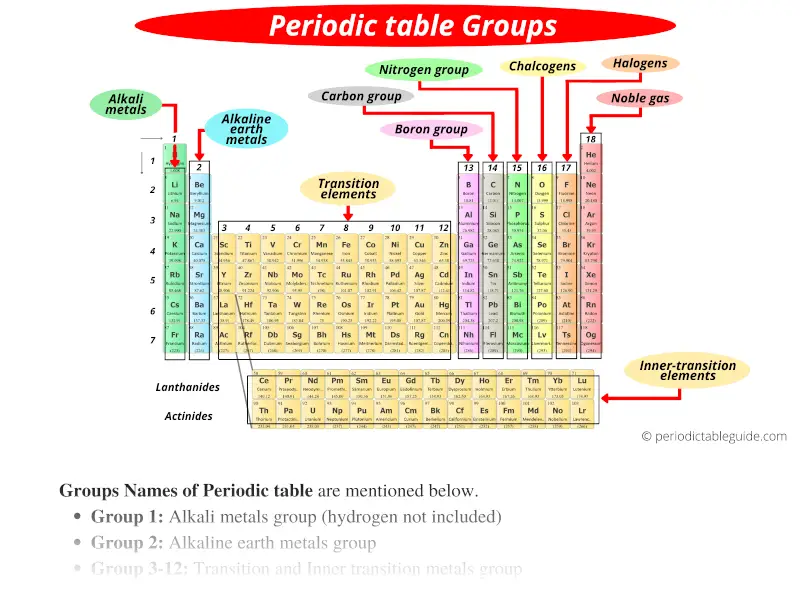
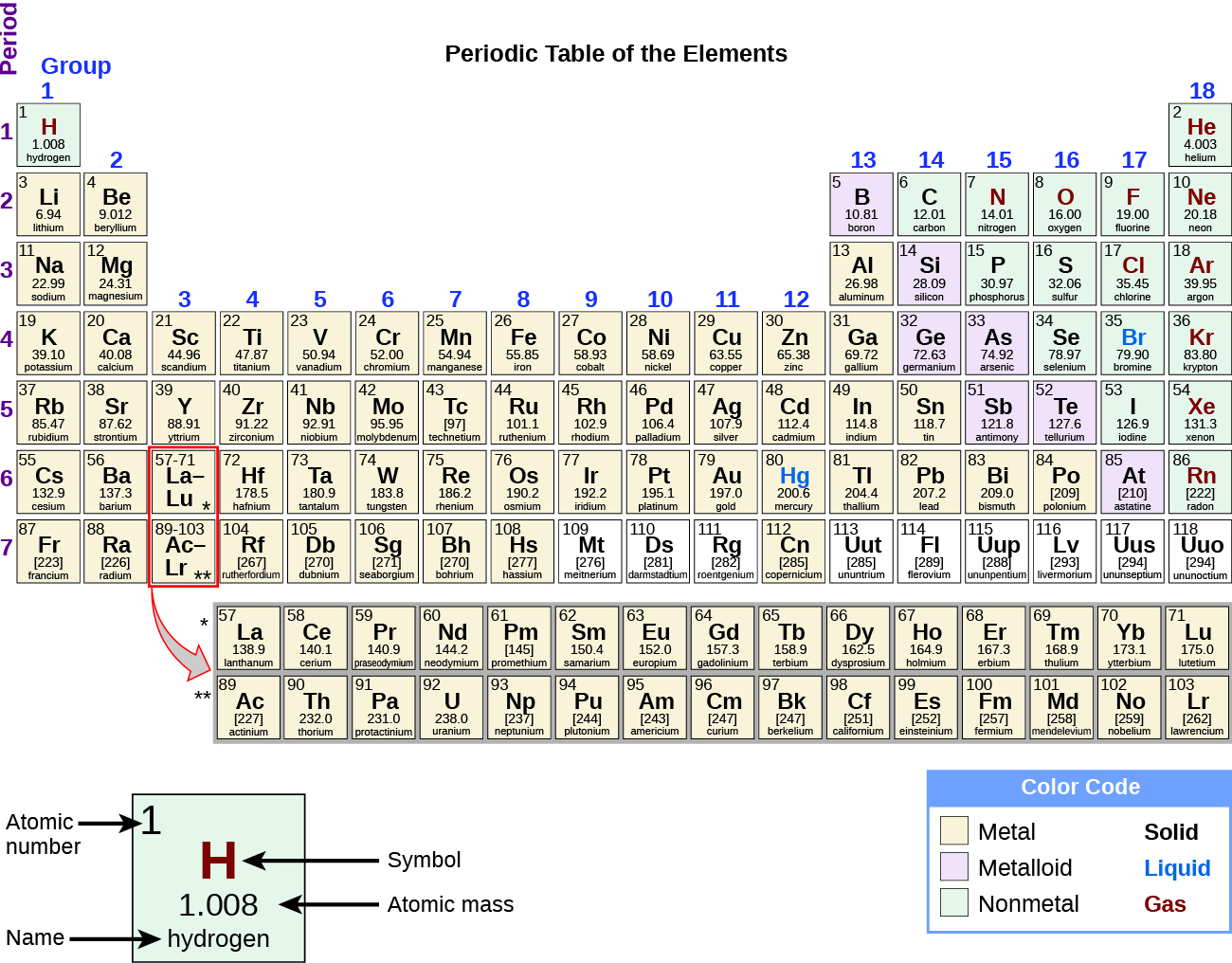
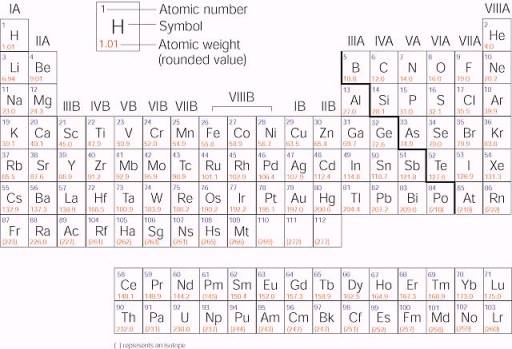
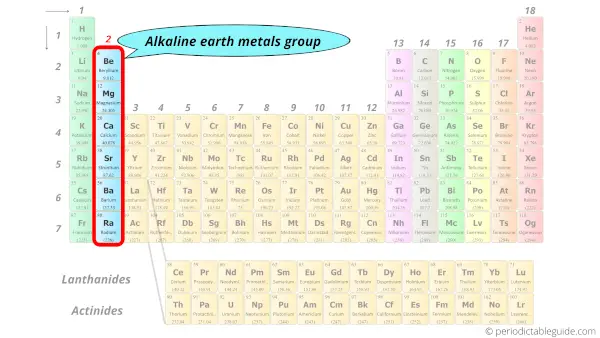
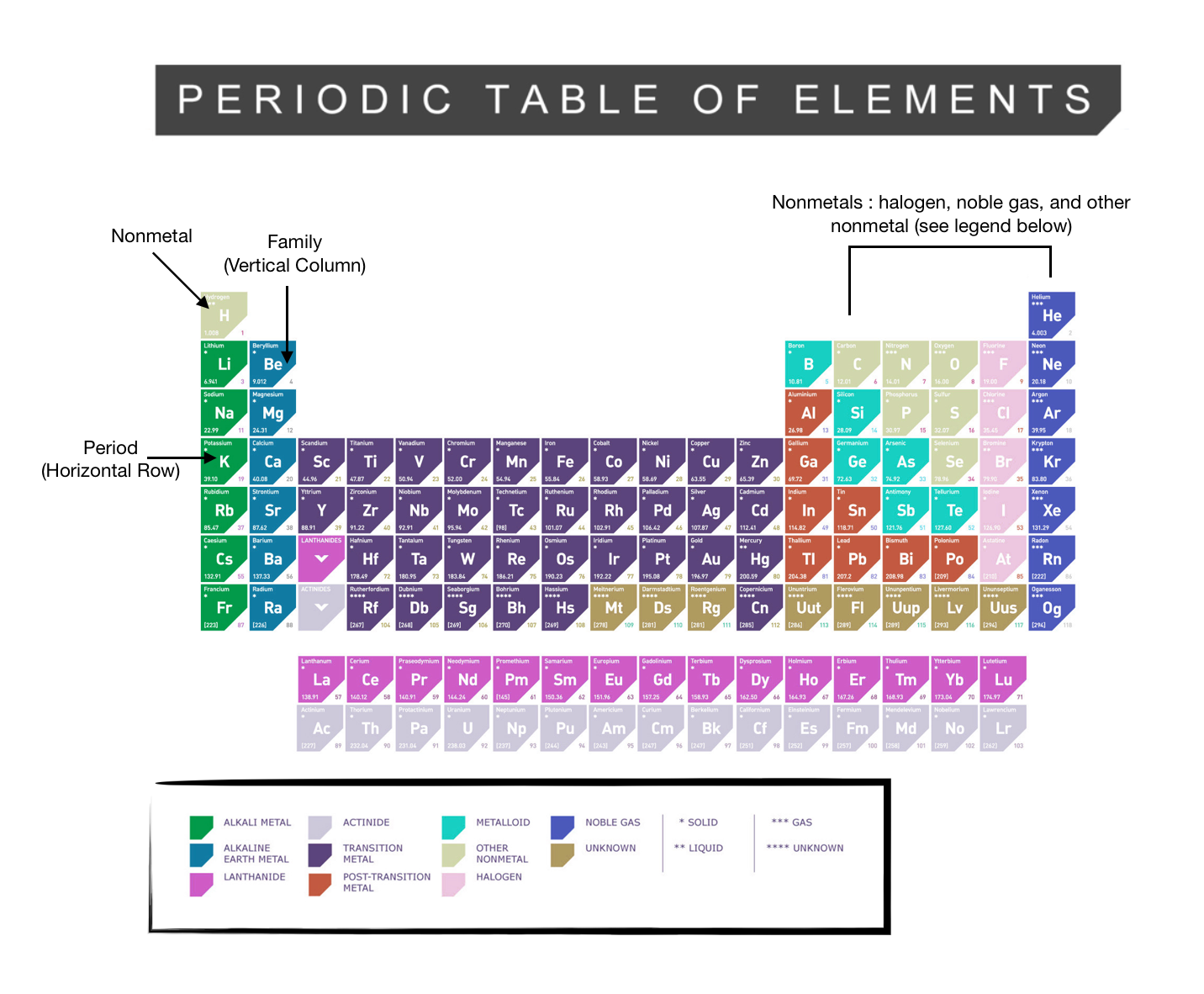
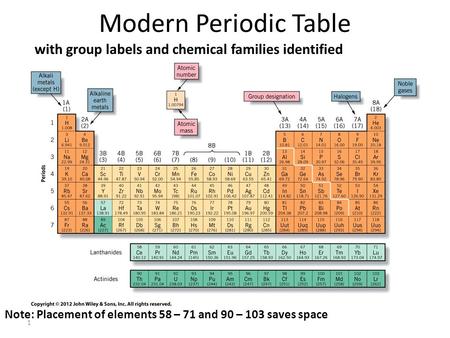
Periodic Table of Elements -Symbols, Atomic Number, Atomic Mass, Groups ... In the periodic table, the vertical columns are called 'groups' and the horizontal rows are called 'periods'. The modern periodic table is based on the modern periodic law put forward by the English physicist Henry Moseley, which states that "the properties of elements are periodic functions of their atomic numbers". Periodic trends in the properties of the elements can be observed down the groups and across the periods of the modern periodic table. Groups and Periods in the Periodic Table - breakingatom.com Common Groups and Periods of the Periodic Table Group 1: The Alkali Metals Group 2: The Alkaline Earth Metals The Transition Metals Group 17: The Halogens Group 18: The Noble Gases Metalloids, the Semi Metals in the Periodic Table The Lanthanides of the Expanded Periodic Table The Actinides of the Expanded Periodic Table The Parts of the Periodic Table - Angelo State University Periods: the horizontal rows on the table, with the elements arranged in order of increasing atomic number. Traditionally, in the United States the taller groups on the table (the main group elements ) are numbered from IA/1A through VIIIA/8A. The shorter groups (the transition metals) are numbered from IB/1B through VIIIB/ 8B.
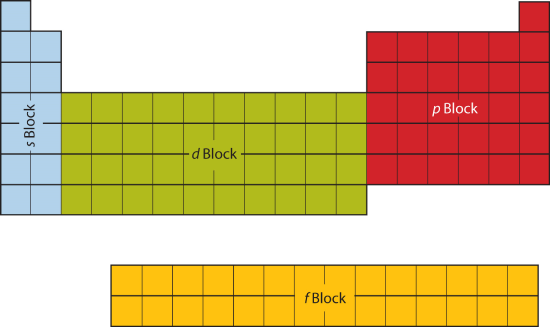
Label these groups of the periodic table.. Blocks of the Periodic Table: s-block, p-block, d-block, f-block The p-Block Elements comprise those belonging to Group 13 to 18 and these together with the s-Block Elements are called the Representative Elements or Main Group Elements. The outer electronic configuration of these elements is ns2 np1. It is interesting to note that the non-metals and metalloids exist only in the p-block of the periodic table. Periodic table labeled with Metals Nonmetals and Metalloids Periodic table labeled with Nonmetals. Elements which are in the top right corner of the Periodic table are classified as Nonmetals ( Hydrogen is also a nonmetal which is located in the group 1). Nonmetals have the tendency to gain the electrons during a chemical reaction. In other words, the elements which gain electrons during a chemical ... The Periodic Table: Families and Periods - dummies The vertical columns of elements are called groups, or families. The most common way the periodic table is classified is by metals, nonmetals, and metalloids. Periods in the periodic table In each period (horizontal row), the atomic numbers increase from left to right. The periods are numbered 1 through 7 on the left-hand side of the table. 2.3: Families and Periods of the Periodic Table Group (family): A vertical column in the periodic table. Alkali metals: Group 1A of the periodic table. Alkaline earth metals: Group 2A of the periodic table. Halogens: Group 7A of the periodic table. Noble gases: Group 8A of the periodic table. Transition elements: Groups 3 to 12 of the periodic table. Contributors
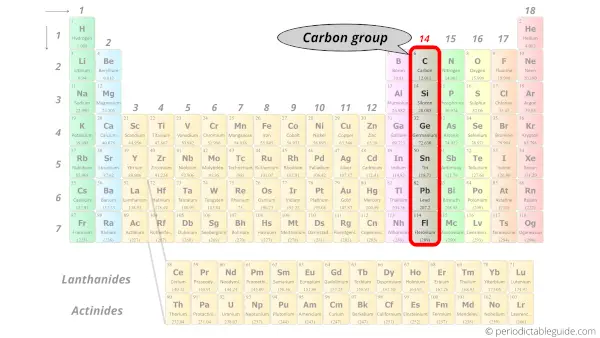
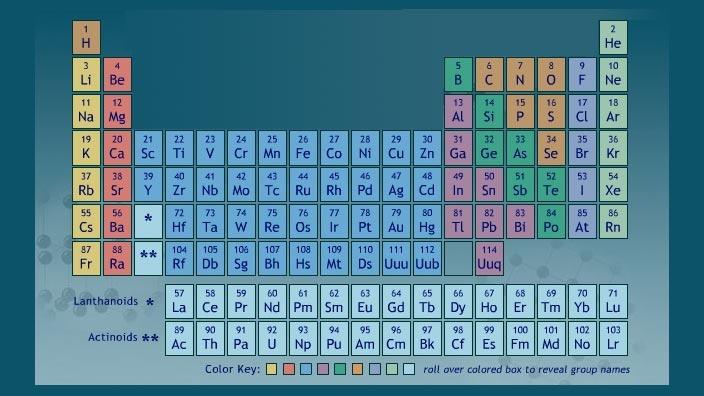
How the Periodic Table groups the elements | Live Science The periodic table of elements is arranged into several broad groups (Image credit: Future) Groups of the Periodic table Alkali metals: The alkali metals make up most of Group 1, the table's first... Labeled Periodic Table of Elements with Name [PDF & PNG] There are 18 groups in the periodic table, which consists of metal and nonmetal. Protons in the tables are positively charged particles. Neutrons are the neutrally negative charge, and electrons are the negative charge particles. It also shows the formation of a bond from one element to the other. PDF Labelled Periodic Table with Charges Solved Label these groups of the periodic table. | Chegg.com Expert Answer. 100% (59 ratings) Transcribed image text: Label these groups of the periodic table. Previous question Next question. Periodic table Groups Explained !! (With 1-18 Group Names) Groups in Periodic Table (With Group Names) There are total 18 different groups in Periodic table. Group 1: Alkali metals group (hydrogen not included) Group 2: Alkaline earth metals group; Group 3-12: Transition and Inner transition metals group; Group 13: Boron group; Group 14: Carbon group; Group 15: Nitrogen group; Group 16: Oxygen group
The Periodic Table Flashcards | Quizlet The horizontal rows in the periodic table are called. periods. How many periods are there in the periodic table. 7. What is the vertical row called in the period table. group. What are the names of the three classes of elements. metal, nonmetal, metalloids. Group 1 (but not H) forms a base when reacting with water. The Periodic Table | CHEM 1305: General Chemistry I—Lecture - Course Hero actinide: inner transition metal in the bottom of the bottom two rows of the periodic table alkali metal: element in group 1 alkaline earth metal: element in group 2 chalcogen: element in group 16 group: vertical column of the periodic table halogen: element in group 17 inert gas: (also, noble gas) element in group 18 Standard periodic table, group labels - Stock Image - C001/3001 Well-known groups include the alkali metals (Group 1), the alkaline earth metals (Group 2), the halogens (Group 17) and the noble gases (Group 18). The standard periodic table has 118 elements arranged in 18 groups and 7 periods. Each element is represented by its chemical symbol. Above each symbol is the element's atomic number, and below it ... Label these groups of the periodic table. | Chegg.com Science. Chemistry. Chemistry questions and answers. Label these groups of the periodic table.
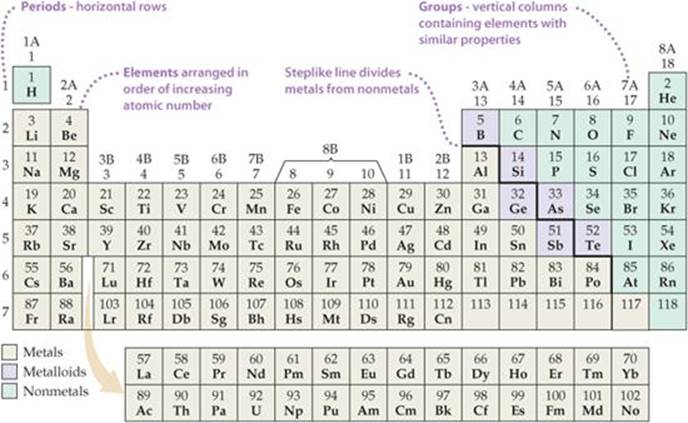
3.2: Organization of the Periodic Table - Chemistry LibreTexts The elements are arranged in seven horizontal rows, called periods or series, and 18 vertical columns, called groups. Groups are labeled at the top of each column. In the United States, the labels traditionally were numerals with capital letters. However, IUPAC recommends that the numbers 1 through 18 be used, and these labels are more common.
An Element In Group 2 Of The Periodic Table An Element In Group 2 Of The Periodic Table. Pauli Exclusion Basic principle The periodic law states that every compound elements possess a normal design of components. Mendeleev initial stated this rules in 1869, and the Pauli exclusion principle provided crucial theoretical help for this thought.
Periodic Table: Groups 1, 2, 13, 14, 15, 16, 17, and 18 - Quizlet Cesium, Group 1. Fr. Francium, Group 1. Be. Beryllium, Group 2. Mg. Magnesium, Group 2. Ca. Calcium, Group 2.
The Parts of the Periodic Table - Angelo State University Periods: the horizontal rows on the table, with the elements arranged in order of increasing atomic number. Traditionally, in the United States the taller groups on the table (the main group elements ) are numbered from IA/1A through VIIIA/8A. The shorter groups (the transition metals) are numbered from IB/1B through VIIIB/ 8B.
Groups and Periods in the Periodic Table - breakingatom.com Common Groups and Periods of the Periodic Table Group 1: The Alkali Metals Group 2: The Alkaline Earth Metals The Transition Metals Group 17: The Halogens Group 18: The Noble Gases Metalloids, the Semi Metals in the Periodic Table The Lanthanides of the Expanded Periodic Table The Actinides of the Expanded Periodic Table
Periodic Table of Elements -Symbols, Atomic Number, Atomic Mass, Groups ... In the periodic table, the vertical columns are called 'groups' and the horizontal rows are called 'periods'. The modern periodic table is based on the modern periodic law put forward by the English physicist Henry Moseley, which states that "the properties of elements are periodic functions of their atomic numbers". Periodic trends in the properties of the elements can be observed down the groups and across the periods of the modern periodic table.






:max_bytes(150000):strip_icc()/PeriodicTableCharge-WBG-56a12db23df78cf772682c37.png)











Post a Comment for "38 label these groups of the periodic table."