41 2 step reaction energy diagram
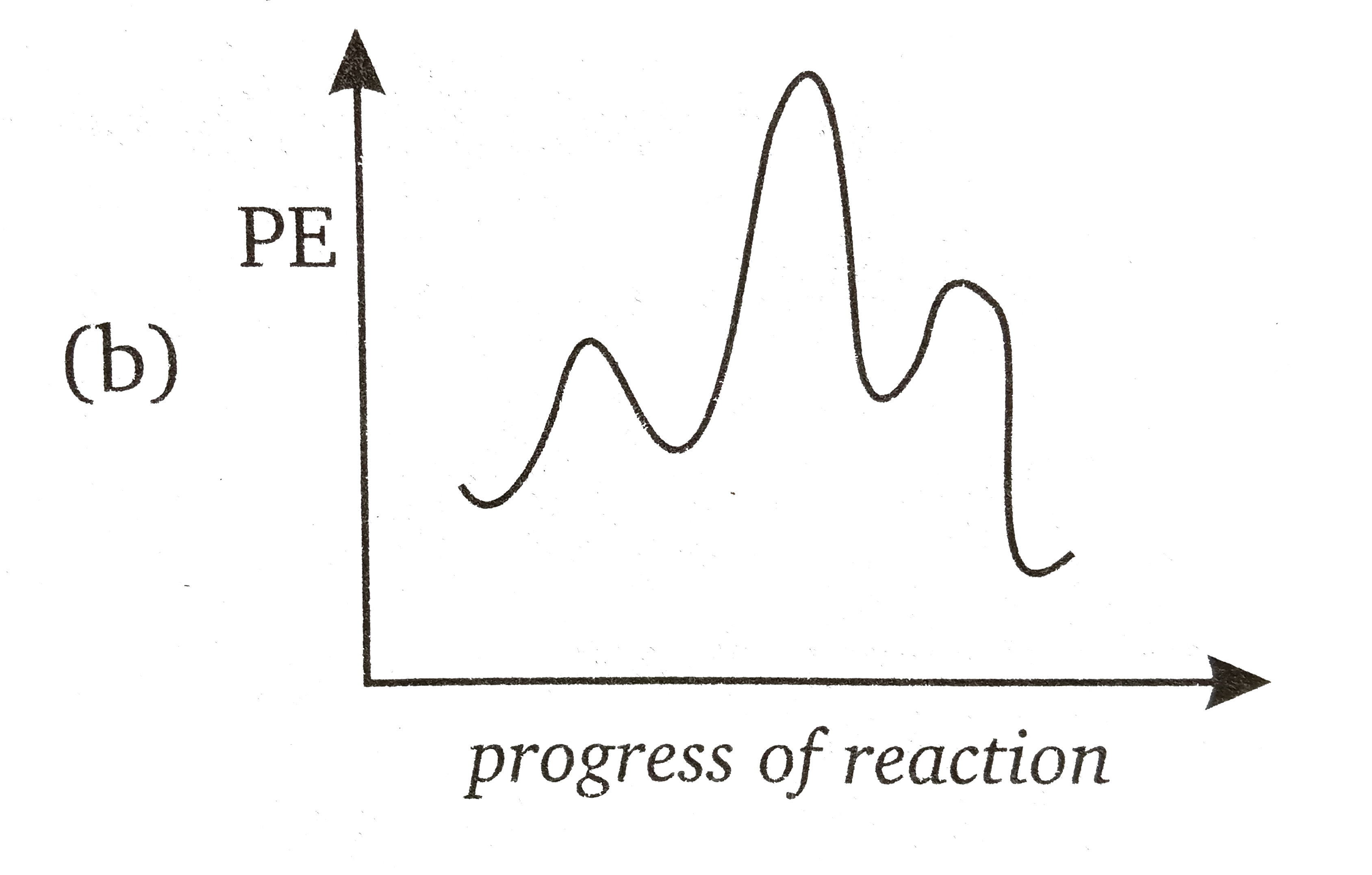
The reaction-energy diagram for a two step endothermic reaction with a ... Interpretation: The reaction-energy diagram for a two step endothermic reaction with a rate-limiting second step is to be drawn. Concept introduction: The graphical representation of chemical reaction in which x-axis represents energy of the reaction and y-axis represents the extent of reaction process is called energy profile diagram. The reaction is exothermic or endothermic can be predicted ... 6.8: Energy Diagram for a Two-Step Reaction Mechanism A potential energy diagram for an S N 1 reaction shows that the carbocation intermediate can be visualized as a kind of valley in the path of the reaction, higher in energy than both the reactant and product but lower in energy than the two transition states. Exercise. Draw structures representing TS1 and TS2 in the reaction above.
Solved Choose the energy diagram for a two step reaction | Chegg.com This problem has been solved! See the answer. Choose the energy diagram for a two step reaction A-->B-->C, in which the relative energy of the compounds is AB is rate determining. Show transcribed image text.
2 step reaction energy diagram
The roles of oxygen vacancies in electrocatalytic oxygen ... Jul 01, 2020 · Calculated free energy diagram for OER over (a) pristine Co 3 O 4 and (b) Co 3 O 4 with oxygen vacancies (V O – Co 3 O 4). The step at the red cycle region is the rate-determining step (RDS). Reproduced from Ref. with permission from the Royal Society of Chemistry. (For interpretation of the references to colour in this figure legend, the ... Energy Diagrams of Two Step Reactions - YouTube Watch Complete videos @ Organic Chemistry 1 Answered: Draw a reaction-energy diagram for a… | bartleby Draw a reaction energy diagram for a two-step exothermic reaction whose second step is faster than the first step. Label the intermediate, transition states, ΔH, and activation energies. arrow_forward Sketch a qualitative reaction energy diagram for a chemical reaction with and without a catalyst. Assume the uncatalyzed reaction is endothermic.
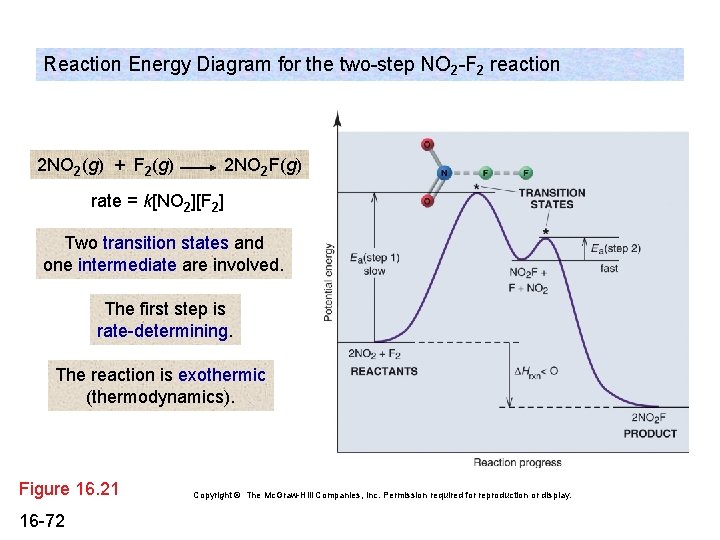
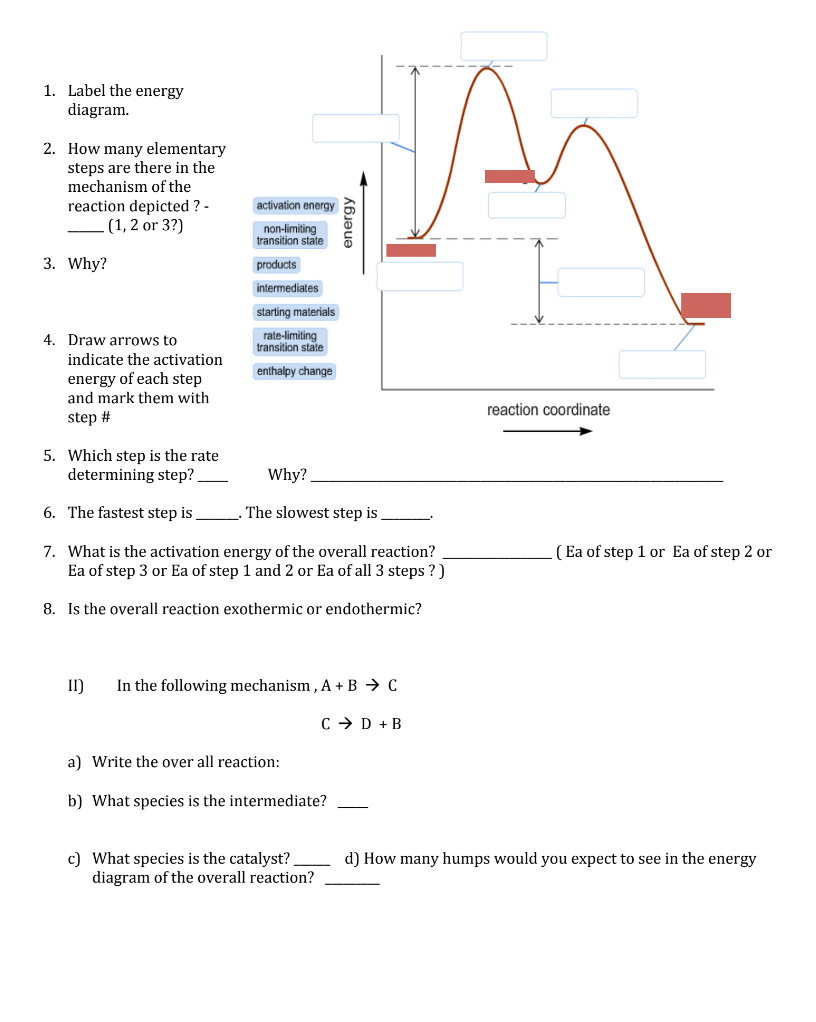
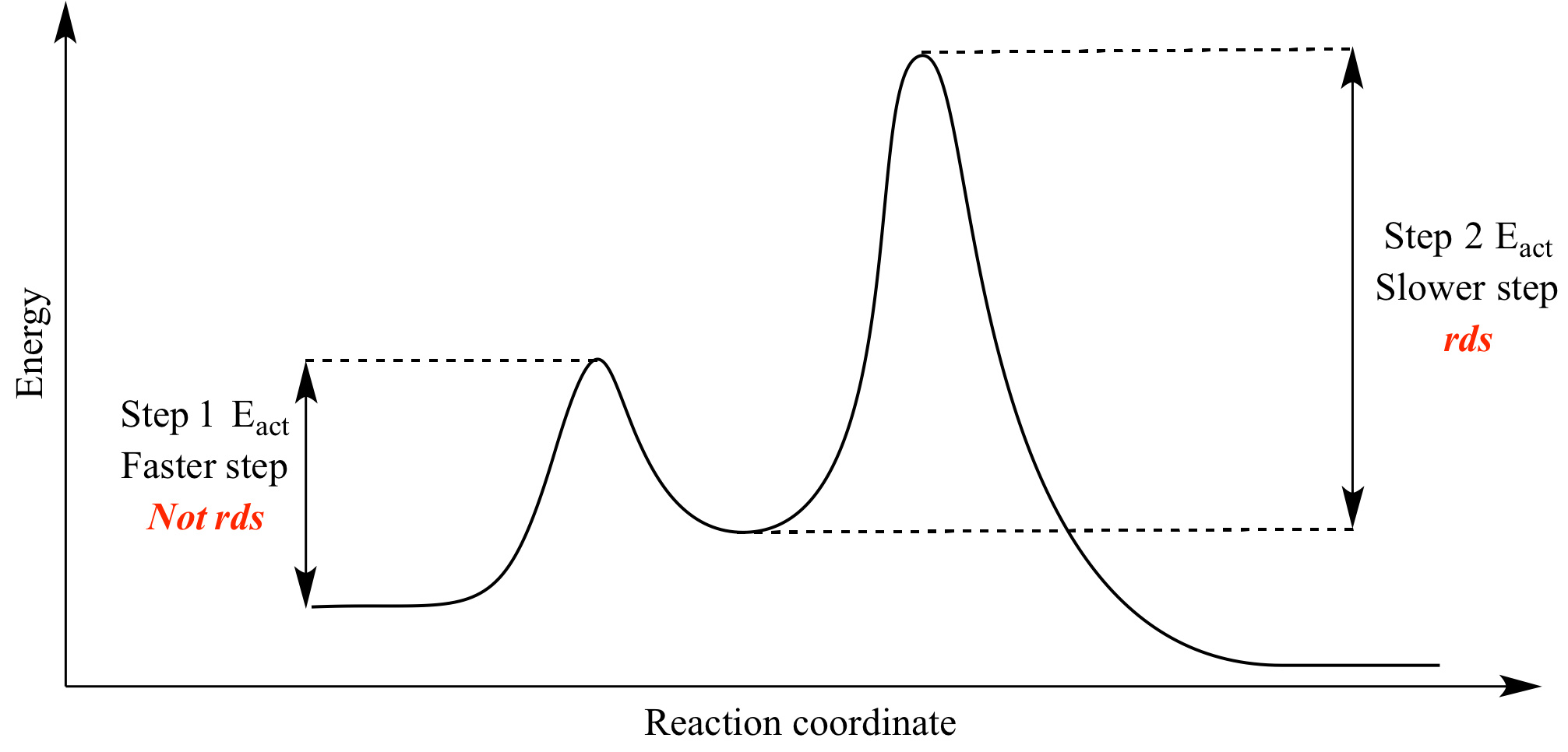
2 step reaction energy diagram. Energy Diagram for a Two-Step Reaction Mechanism Complete Energy Diagram for Two-Step Reaction A Two-Step Reaction Mechanism The transition states are located at energy maxima. The reactive intermediate B+ is located at an energy minimum. Each step has its own delta H and activation energy. The overall energy difference between the starting materials and products is delta H overall. CH 368: Unit 2 - research.cm.utexas.edu The transition state of a reaction step is considered to be the highest energy point on the minimum (free) energy reaction path (MERP). Reactions are considered to occur via the path from reactants to products which is of lowest energy. The TS is the point of highest energy on this path. As such it represents a saddle point on the free energy ... Draw an energy diagram for a two-step exergonic reaction ... - Numerade That means that the activation energy, the second step Yes, last time, the activation energy of the first. Okay, so, no, that's represent, that's and on energy diagram. Okay, so we have two steps that we need to be accountable. So now let's just start 12 with the reactions. You know, that's draw the first step of the action. 6.15: Energy Diagram for a Two-Step Reaction Mechanism Recall that the first step of the reaction above, in which two charged species are formed from a neutral molecule, is much the slower of the two steps, and is therefore rate-determining. This is illustrated by the energy diagram, where the activation energy for the first step is higher than that for the second step.
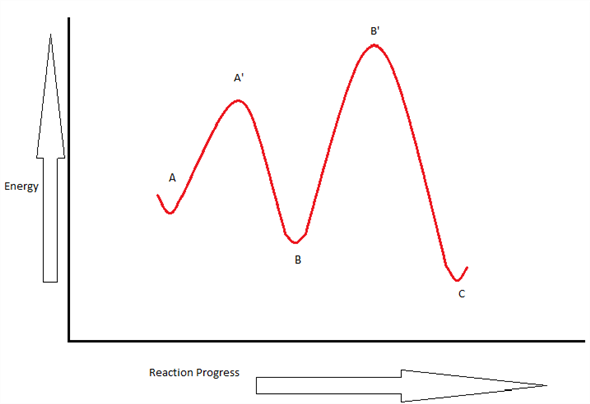
7.2 SN2 Reaction Mechanisms, Energy Diagram and Stereochemistry Energy Diagram of the SN2 Mechanism. The energy changes for the above reaction can be represented in the energy diagram shown in Fig. 7.1. S N 2 is a single-step reaction, so the diagram has only one curve. The products CH 3 OH and Br - are in a lower energy than the reactants CH 3 Br and OH -, which indicates that the overall reaction is ... Multistep reaction energy profiles (video) | Khan Academy Many chemical reactions have mechanisms that consist of multiple elementary steps. The energy profile for a multistep reaction can be used to compare the activation energies of the different steps and identify the rate-determining step. The energy profile can also be used to determine the overall change in energy for the reaction. Cellular Respiration Equation, Types, Stages, Products & Diagrams Dec 07, 2021 · C 6 H 12 O 6 → 2 CH 3 CH(OH)COOH + 2 CO 2 + 2 ATP; On the other hand, microorganisms like yeast produce ethanol and carbon dioxide. Such process is referred to as the ethanol or alcohol fermentation. C 6 H 12 O 6 → 2 C 2 H 5 OH + 2 CO 2 + 2 ATP; In both types of fermentation process, only 2 ATP are produced from a glucose molecule. Answered: Draw an energy diagram for a two-step… | bartleby Science Chemistry Q&A Library Draw an energy diagram for a two-step reaction, A → B → C, where the relative energy of these compounds is C < A < B, and the conversion of B → C is rate-determining.
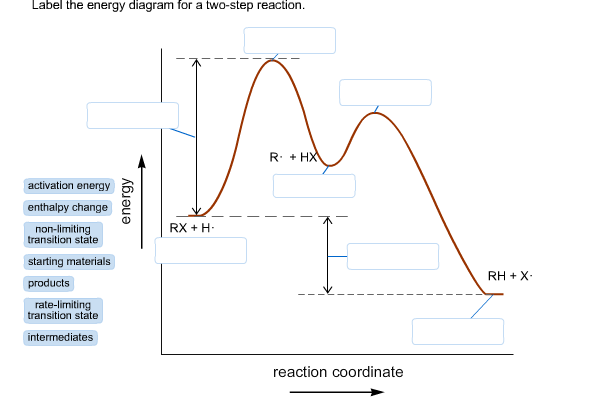
7.4 SN1 Reaction Mechanisms, Energy Diagram and Stereochemistry Figure 7.4a Energy diagram for SN1 reaction between (CH3)3CBr and H2O. The connection between the first two curves represent the carbocation intermediate. Generally, the intermediate is the product of one step of a reaction and the reactant for the next step. The intermediate is at a relatively lower energy level compared to the transition ... Solved Label the energy diagram for a two-step reaction. - Chegg Question: Label the energy diagram for a two-step reaction. enthalpy change transition state starting materials RX+H products rate-limiting transition state intermediates activation energy reaction coordinate This problem has been solved! See the answer Show transcribed image text Expert Answer 100% (54 ratings) PDF Potential energy diagram for a two-step sequential reaction Potential energy diagram for a two-step sequential reaction A 1 19 3 50 AB* 4 33 3 40 B 3 28 3 30 BC* 4 41 3 20 C 5 13 3 10 3 0 the red lines are error bars on the x -value Adjust energies Derived from Scott Sinex's Excel chart 1 2 3 4 5 0 10 20 30 40 50 Energy Reaction coordinate step 1 step 2 A B C A B C k 1k 2 Mechanisms and Potential Energy Diagrams - Course Hero A potential energy diagram for a two-step reaction is shown and labeled. Practice View the section on two-step reactions at the site below and then do the self-test (both buttons are at the top of the slide). Don't worry about - just consider it an indication of activation energy as is in the diagram above.
Chapter 8, pages 5 and 6 - St. Edward's University Energy Diagram for an E2 Reaction: Since all bonds are broken and formed simultaneously, the E2 reaction occurs in one single step in very similar fashion to the S N 2 reaction. The energy diagram for an E2 reaction and an S N 2 reaction look identical to each other. As the base approaches the hydrogen and bonds begin to form and break, the ...
Energy Diagrams: Describing Chemical Reactions Draw an energy diagram for a two-step reaction that is exothermic overall, and consists of a fast but endothermic first step, and a slow but exothermic second step. Indicate DGrxn, as well as DG1* and DG2* for the first and second activation energies, respectively. Label the positions corresponding to the transition states with an asterisk.
Draw a reaction coordinate diagram for a two-step reaction i - Quizlet Draw a reaction coordinate diagram for a two-step reaction in which the first step is endergonic, the second step is exergonic, and the overall reaction is endergonic. Label the reactants, products, intermediates, and transition states. Explanation Verified Reveal next step Reveal all steps Create a free account to see explanations
Multistep Reactions - Softschools.com The energy diagram of a two-step reaction is shown below. In the above reaction, a reactant goes through one elementary step with a lower activation energy (transition state 1) to form the intermediate. The intermediate then goes through a second step (transition state 2) with the highest energy barrier to form the product.
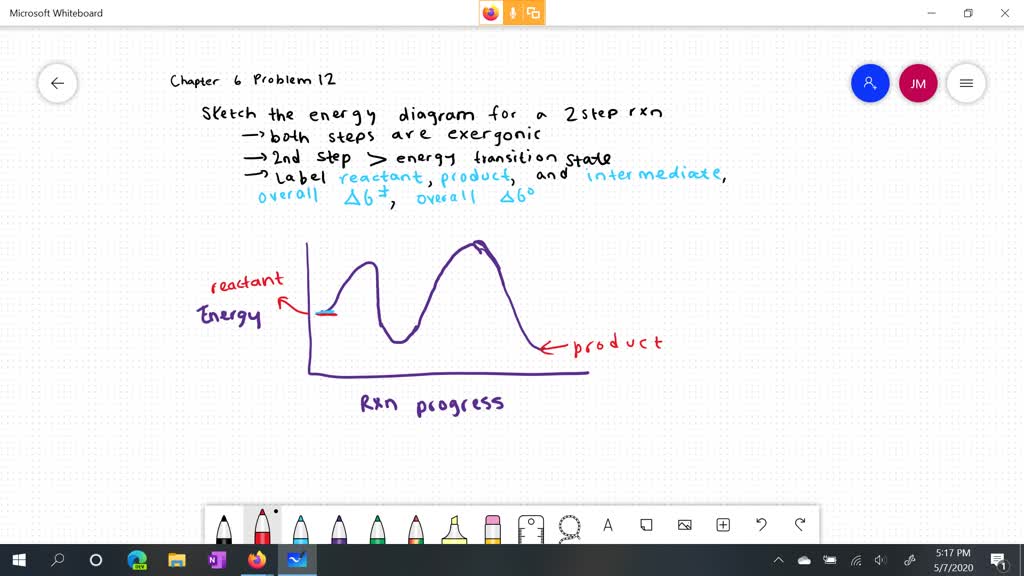
Solved: Sketch an energy diagram for a two-step reaction in which ... Sketch an energy diagram for a two-step reaction in which both steps are exergonic and in which the second step has a higher-energy transition state than the first. Label the parts of the diagram corresponding to reactant, product, intermediate, overall Δ G‡, and overall Δ G °. Step-by-step solution 91% (11 ratings) for this solution Step 1 of 3
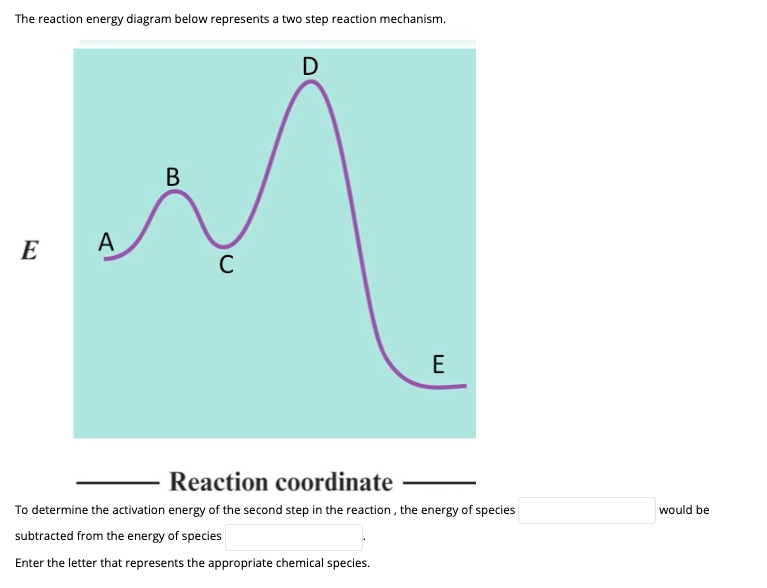
CHEM 112B - L4 - Integrated Rate Laws (Notes).pdf Garner - Chem 112B - Lecture 3 Consider the reaction energy diagram for a two-step reaction shown below. Which step is the rate-determining step for this reaction? Rate-limiting step = step with higher E a 1 A. Step 1 B. Step 2 E a (step 1) E a (step 2)
Potential Energy Diagrams - Chemistry - Catalyst, Endothermic ... - YouTube This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f...
Draw a reaction-energy diagram for a two-step endothermic re | Quizlet Find step-by-step Chemistry solutions and your answer to the following textbook question: Draw a reaction-energy diagram for a two-step endothermic reaction with a rate-limiting second step..
Draw an energy diagram for a two-step reaction, $$ A \righ | Quizlet Find step-by-step Chemistry solutions and your answer to the following textbook question: Draw an energy diagram for a two-step reaction, $$ A \rightarrow B \rightarrow C, $$ where the relative energy of these compounds is C < A < B, and the conversion of $$ B \rightarrow C $$ is rate-determining..
Energy Diagrams of Reactions | Fiveable To find the activation energy, you should be looking for two numbers: the potential energy of the reactants and the energy of the activated complex (the maximum point). (energy of activation complex) - (PEreactants) (100 kJ) - (40 kJ) = 60 kJ In other words, it takes 60 kJ of energy to complete the reaction.
Answered: Draw a reaction energy diagram for a… | bartleby Science Chemistry Q&A Library Draw a reaction energy diagram for a two-step exothermic reaction whose second step is faster than the first step. Label the intermediate, transition states, ΔH, and activation energies.
Answered: Draw a reaction-energy diagram for a… | bartleby Draw a reaction energy diagram for a two-step exothermic reaction whose second step is faster than the first step. Label the intermediate, transition states, ΔH, and activation energies. arrow_forward Sketch a qualitative reaction energy diagram for a chemical reaction with and without a catalyst. Assume the uncatalyzed reaction is endothermic.
Energy Diagrams of Two Step Reactions - YouTube Watch Complete videos @ Organic Chemistry 1
The roles of oxygen vacancies in electrocatalytic oxygen ... Jul 01, 2020 · Calculated free energy diagram for OER over (a) pristine Co 3 O 4 and (b) Co 3 O 4 with oxygen vacancies (V O – Co 3 O 4). The step at the red cycle region is the rate-determining step (RDS). Reproduced from Ref. with permission from the Royal Society of Chemistry. (For interpretation of the references to colour in this figure legend, the ...







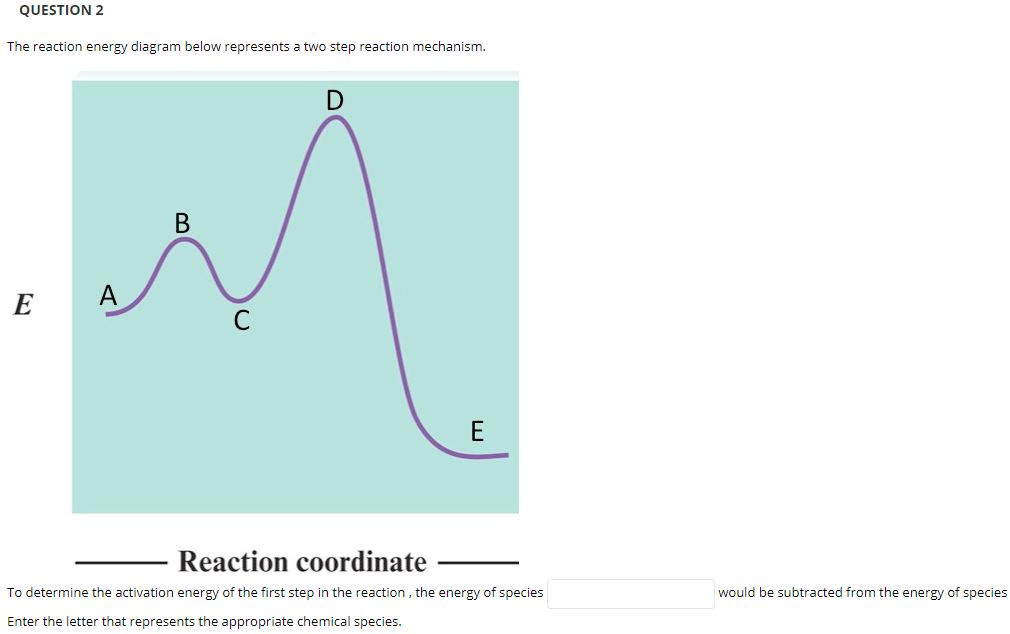

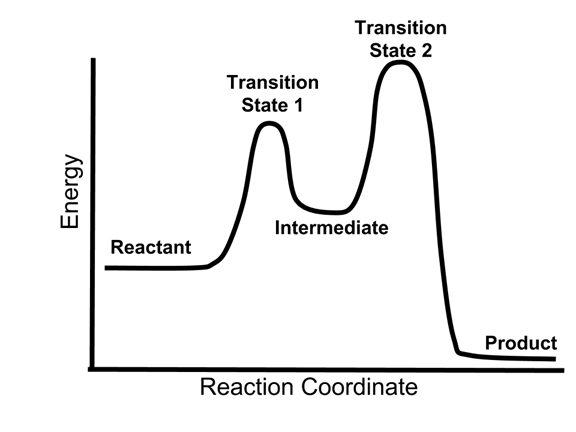




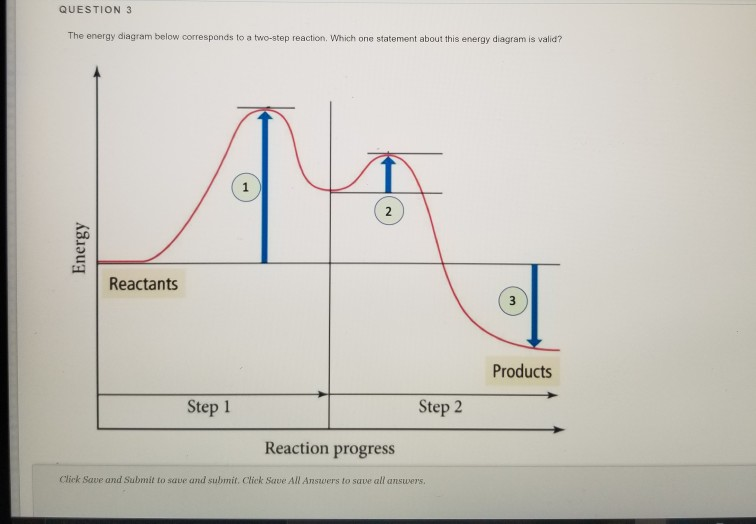
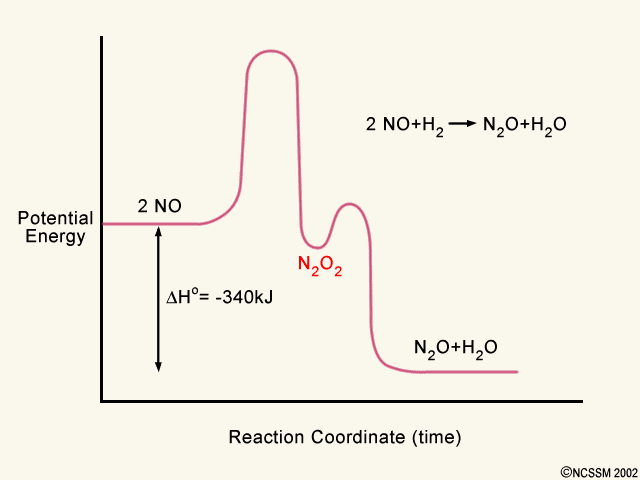









Post a Comment for "41 2 step reaction energy diagram"